BIOCHEMISTRY, BIOTECHNOLOGY AND DIAGNOSTICS
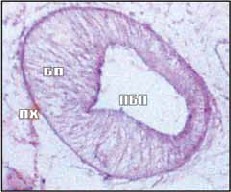
The purpose of the research is to study the morphofunctional organization, histological and histochemical features of the digestive system of the marita Parafasciolopsis fasciolaemorpha, which provide adaptation to parasitism in the endostasis – the liver Alces alces.
Materials and methods. Mature specimens of the trematode P. fasciolaemorpha (Ejsmont, 1932) collected from the bile ducts of the Alces alces liver served as the material. Maritas were fixed in 70 and 80% alcohols, Schaffer alcohol-formalin 1 : 9, and 10% neutral formalin. Histological stains: with hematoxylin-eosin and by the Mallory method, followed by additional staining of the nuclei with Orta lithium carmine. Histochemical stains: sublimate-bromophenol blue according to Bonheg, Schick reaction according to McManus with additional staining of nuclei with Mayer's hemalaune, alcian blue according to Steedman and Mowry (pH 3.0 and 2.2) and toluidine blue (pH 2.0-5.0).
Results and discussion. Parafasciolopsis fasciolaemorpha (Ejsmont, 1932) is the most pathogenic hepatoparasite of Alces alces L. The study of the features of the morphological organization of the digestive system as one of the border systems of the organism of the parasite revealed a number of features: the muscles of the large oral sucker contain many total proteins, which is confirmed by intense bromophenolophilia; in the thickness of the sucker wall there are small secretory cells and neurosecretory cells with abundant alcian- and toluidinophilic vacuolated cytoplasm; the presence of single and grouped digestive glands located in the parenchyma at the junction of the prepharynx to the pharynx, and on the border between the pharynx and the esophagus; in the apical part of the intestinal epithelium, a “brush border” is formed, the thin microvilli of which contribute to an increase in the working surface of parietal digestion in P. fasciolaemorpha, enhancing the trophic processes of the helminth to survive in the body owner. The revealed histological and histochemical features of the parafasciolopsis digestive system can be considered as examples of adaptive specialization at the site of endostasis.
The purpose of the research is a comparative assessment of the effectiveness of methods to diagnose eimeriosis in turkeys and the species identification.
Materials and methods. A comparative effectiveness assessment of life-time diagnostic methods for eimeriosis in turkey poults was conducted using coproscopic examinations: with sodium chloride alone, and with sodium chloride and glycerin according to Darling; with sodium chloride and glucose according to McMaster; and with sodium chloride alone according to Fülleborn. The diagnostic strength of different methods was evaluated with Eimeria oocysts artificially placed in standard litter samples free from infection. Morphological examinations and characteristics determination of Eimeria species in the turkey poults were conducted in the laboratory after the completed sporulation.
Results and discussion. The diagnostic strength of the Fülleborn’s flotation method for turkey eimeriosis was 62.4%, 79.2% for the combined Darling1 methods, 85.6% for the combined Darling2 methods, and 90.4% for the McMaster’s methods. The combined Darling’s and McMaster’s methods used by us provide, according to their technology, for double centrifugation: water settling and flotation with saline, thus the microscopically examined sample contained not so many feed residues or other particles, which affected the diagnostic strength of the method. The young turkeys from the Penza and Moscow Regions’ farms were found to have the following types of Eimeria: Eimeria meleagrimitis in 62–80%, E. meleagridis in 15–16%, E. adenoides in 5–13%, and E. gallopavonis in 9%. E. meleagrimitis and E. meleagridis dominated on the turkey farms in the said regions. E. adenoides and E. gallopavonis were significantly less common.
The purpose of the research is to identify perinatal infection in the dynamics, and assess the number and genetic status of bovine leukemia proviruses isolated from young animals, and correlations between some indicators of the infectious process based on gene diagnostics methods.
Materials and methods. We used the material from cattle of different age groups: 1, calves (30–40 minutes after birth before colostrum and 15 to 45 days); and 2, heifers (not older than two years). Radial immunodiffusion (RID), real-time polymerase chain reaction (PCR), and phylogenetic analysis were used.
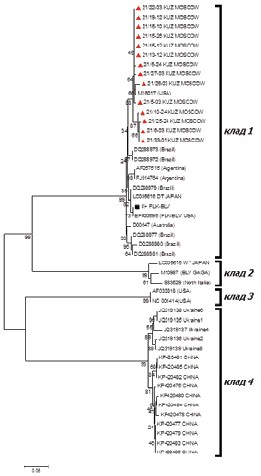
Results and discussion. An assessment is given for perinatal infection of the young cattle. The detection rate of the infection in the calves was 4.15% (PCR) and 1.09% (RID); and 1.1% (PCR) and 0.88% (RID) in the heifers. A 36-fold decrease of the infection was found in positive dynamics (2013–2022) from 14.5 to 0.4% with passing through 0% (2020) and being at the level of 0% (2022). The proviral load ranged from 2.02 × 104 to 8.38 × 106 GE/mL in the blood of the examined animals. The BLV isolates obtained were shown to belong to two genotypes, GIV and GVII (env), and clade 1 (pol). We assessed an overestimation of the number of the proviruses by a factor of three in the animals under two years of age (3.83 × 106GE/mL) relative to that in the 1-month-old calves (1.3 × 106 GE/mL), and by a factor of nine for GIV relative to GVII. It is important to develop gene diagnostics algorithms to increase the effectiveness of routine tools to prevent the spread of this retrovirus infection in young animals at an early stage, which is confirmed by a decrease to 0% of detected retrovirus infection in young animals over time. The provirus number was higher in the heifers than the calves; the proviral load level was higher in the multiparous dairy cows than the nulliparous animals, and quantitative indicators were higher in the animals’ blood with the GIV genotype relative to those with the GVII genetic variant of the BLV.
PATHOGENEZIS, PATHOLOGY AND ECONOMIC DAMAGE
The purpose of the research is to study the effect of syphaciosis on biochemical and clinical blood parameters of outbred rats.
Materials and methods. Outbred male rats weighing 180–200 g were examined for helminth eggs by coproovoscopy and a Scotch tape test using a microscope Micromed 1 ver. 2-20. A biochemical blood assay was conducted on a Beckman Coulter DxC 700AU analyzer (USA), and a haematology test panel was made on a PCE 90-Vet analyzer (USA). Fenbendazole was used for preventive dehelminthization. Statistical processing was performed using the software Studet200.
Results and discussion. The study results on biochemistry and hematology of the outbred rats’ blood showed a significant decrease in LDH levels and an increase in hematocrit in the animals infected with Syphacia spp.
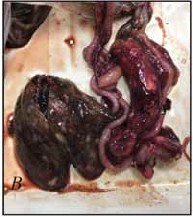
The purpose of the research is to evaluate the parasitism effects on clinical parameters of laboratory animals.
Materials and methods. The venous blood of 20 rabbits aged 1 year was taken to perform biochemical and general blood tests, for which purpose hematological analyzers were used. The animals were dissected using the Shor’s method. The topography of the organ complex was evaluated, and pathological material was collected and placed to 10% buffered formalin. For pathomorphological examination, liver, spleen, lung, and kidney samples were taken. To determine the histological pattern, paraffin-embedded samples on Thermo Scientific semi-automatic equipment were used. Histologic specimens were stained with hematoxylin and eosin. The histoarchitecture of the specimens was evaluated using an Axio A1.0 microscope, and photography was conducted with the AxioVision software.
Results and discussion. The article presents the data of the general and biochemical blood tests of the blood from the rabbits intended to be used in the experiment. Further, the pathoanatomical picture of the liver was shown in animals infected with Eimeria spp., and the histological pattern was presented for parenchymal organs. We found that the main biochemical values that exceeded reference values were liver values, namely, aspartate aminotransferase and alanine aminotransferase. We also observed an increase in monocytes and granulocytes in the blood. Pathological and anatomical changes were only expressed in the liver, while no changes in the macro pattern were observed in other organs. Histological examination of parenchymal organs showed a significant pathology in the liver due to endogenous stages of oocysts occurred in its structure. Additionally, we observed a strong eosinophil response in the spleen and a high content of eosinophils in the pulmonary veins.
PHARMACOLOGY, TOXICOLOGY
The purpose of the research is to study the elimination period of Ivermectin residuals after three oral administrations of Iverbutan to broiler chickens.
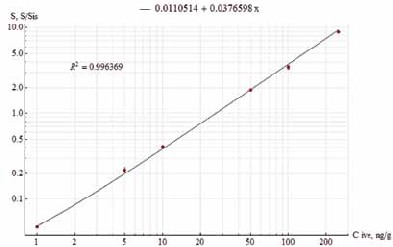
Materials and methods. For the experiment, 18 broiler chickens aged 28 days were selected. The birds were kept in the conditions of the Podolsk Experimental Production Department of the VNIIP – FSC VIEV. Iverbutan contains 0.4% of ivermectin and 10.0% of butaphosphan as active substances. The drug was administered orally by the group method at the rate of 1.0 mL of Iverbutan per 1 Liter of drinking water. Iverbutan was given three times: twice with a 24-hour interval and once after 14 days. The birds were killed and samples of their organs and tissues were taken at 9, 14, and 19 days after the drug was administered three times. Organs and tissues of each following type were collected from 6 birds: muscles, liver, kidneys, and skin with subcutaneous adipose tissue. The technique was based on the determination of Ivermectin by high performance liquid chromatography with modified pre-column accomplished with N-methylimidazole and trifluoroacetic anhydride, followed by fluorescence detection. The quantification was performed by the internal standardization.
Results and discussion. The period was studied for elimination of Ivermectin residuals from the chickens’ body after the drug was administered three times. The residuals that exceed the maximum allowable levels were determined at 9 and 14 days after the drug was completed. It was found that the chickens’ organs and tissues did not contain Ivermectin at 19 days. Thus, poultry meat can be used for food at 19 days after Iverbutan is administered three times.
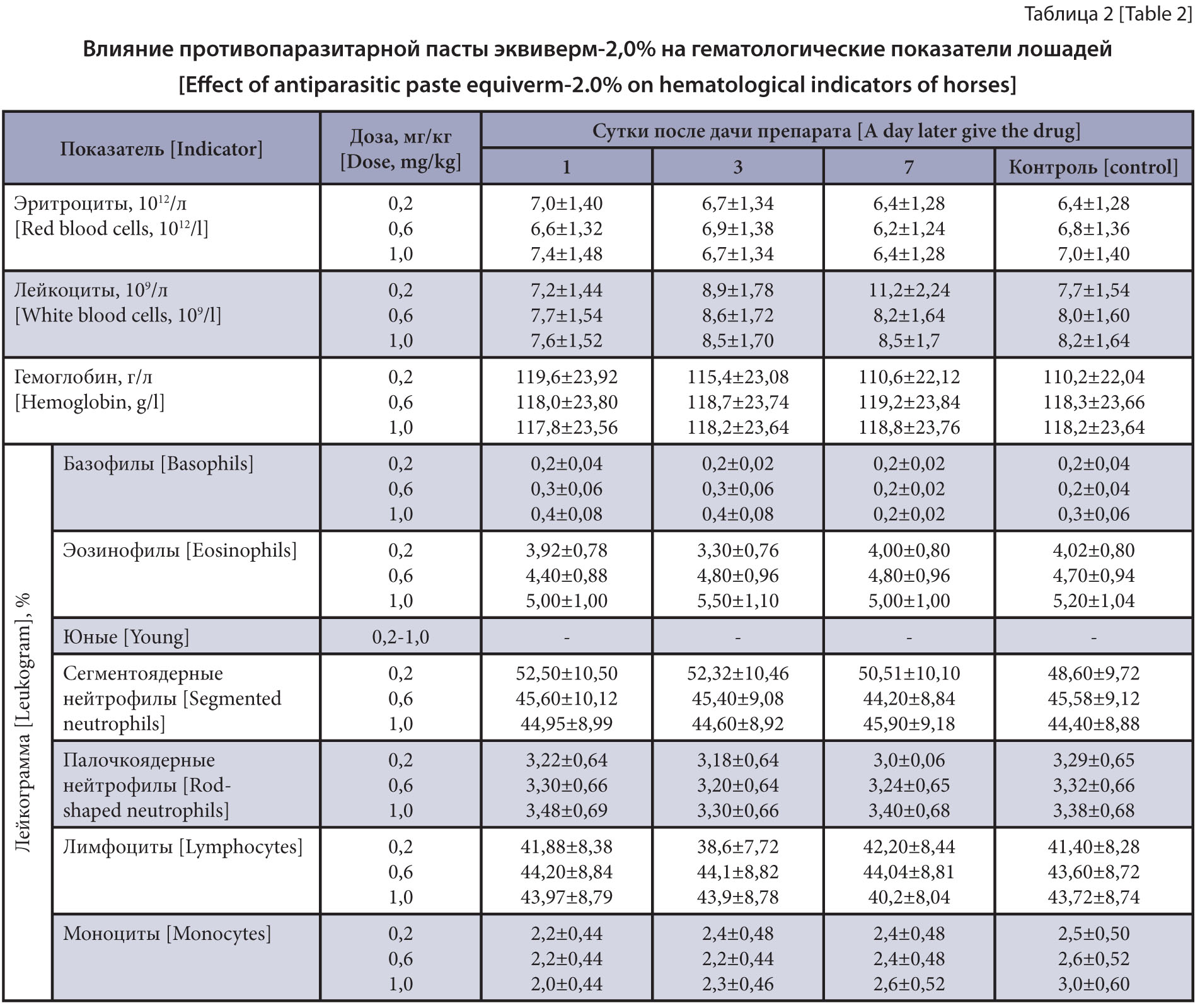
The purpose of the research is to study the effect of the new antiparasitic 2.0% Equiverm in high doses on the clinical state of horses.
Materials and methods. The experiment was conducted on 15 two-year-old crossbred horses weighing up to 300 kg spontaneously infected with Strongylata. To determine the effect of the antiparasitic paste on the horses, three groups of five horses each were formed. The first group of the horses was administered 2.0% Equiverm at a therapeutic dose; the second, at a three-fold increased dose, and the third, at a five-fold increased dose (0.2; 0.6 and 1.0 mg/kg for the active substance (AS), and 1.0; 3.0 and 5.0 mL per 100 kg of body weight for the drug). The horses’ clinical state was studied using standard methods. Blood samples for the study were taken from the jugular vein before the drug on the first, third and seventh days. The results obtained were statistically processed using the computer tool Microsoft Excel 2007.
Results and discussion. It was found that the antiparasitic paste 2.0% Equiverm had no negative effect on clinical, hematological or biochemical parameters after a single oral administration at a therapeutic, three- and five-fold increased dose (0.2; 0.6 and 1.0 mg/kg for the AS, and 1.0; 3.0 and 5.0 mL per 100 kg of the body weight for the drug).
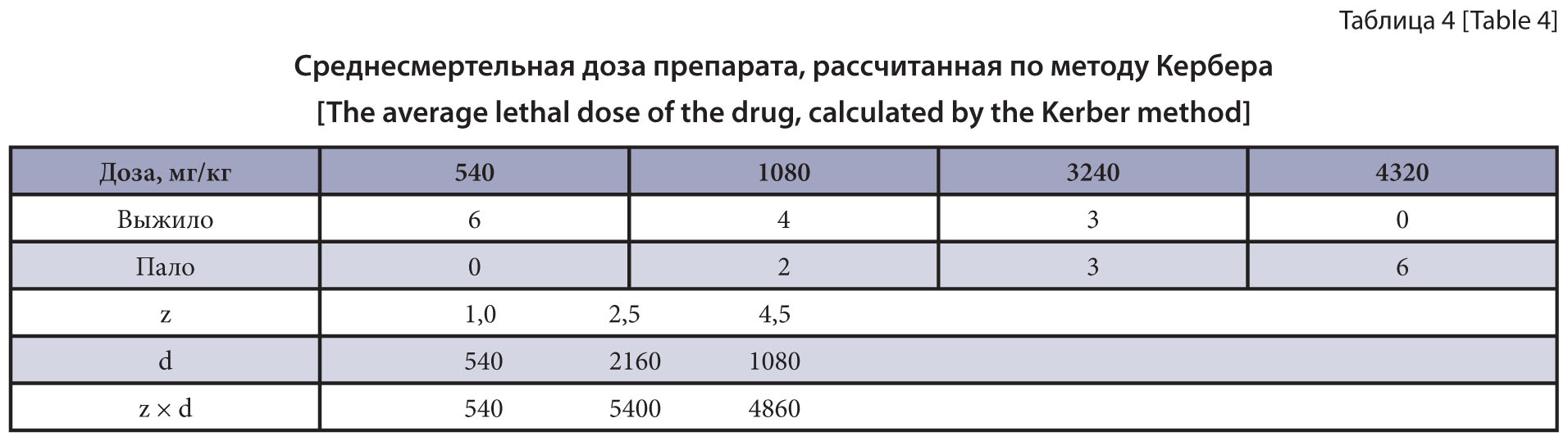
The purpose of the research is the study of acute oral and dermal toxicity, and cumulative properties of the combined drug based on imidacloprid, pyriproxyfen and moxidectin on mice and rats.
Materials and methods. The studies were conducted as provided by the Guidelines of the State Pharmacological
Committee, in the VNIIP – FSC VIEV vivarium in 2021. We studied the acute oral and dermal toxicity, as well as cumulative properties of the combined drug in the form of a solution for external use that contains imidacloprid, pyriproxyfen and moxidectin as active substances. Outbred male mice and male rats were used in studying toxicological characteristics of the drug. General methods were used in studying the acute oral toxicity in the mice and rats, acute dermal toxicity in the rats and cumulative properties of the prototype product in the mice.
Results and discussion. The LD 50 of the prototype product was 800 mg/kg of the animal weight when administered orally to the mice, and 2520±916.7 mg/kg, to the rats. Subject to the established median lethal doses, the drug was classified as the 3rd hazard class according to the general hygienic classification (GOST 12.1.007-76). When studying the acute dermal toxicity in the rats, the LD50 of the drug exceeded the maximum possible dose of 10,000 mg/kg. According to the general hygienic classification (GOST 12.1.007-76), the drug was classified as the 4th hazard class. The accumulation factor was 8.25, in which case the drug can be classified as the group of substances with weak cumulative activity.
TREATMENT AND PREVENTION
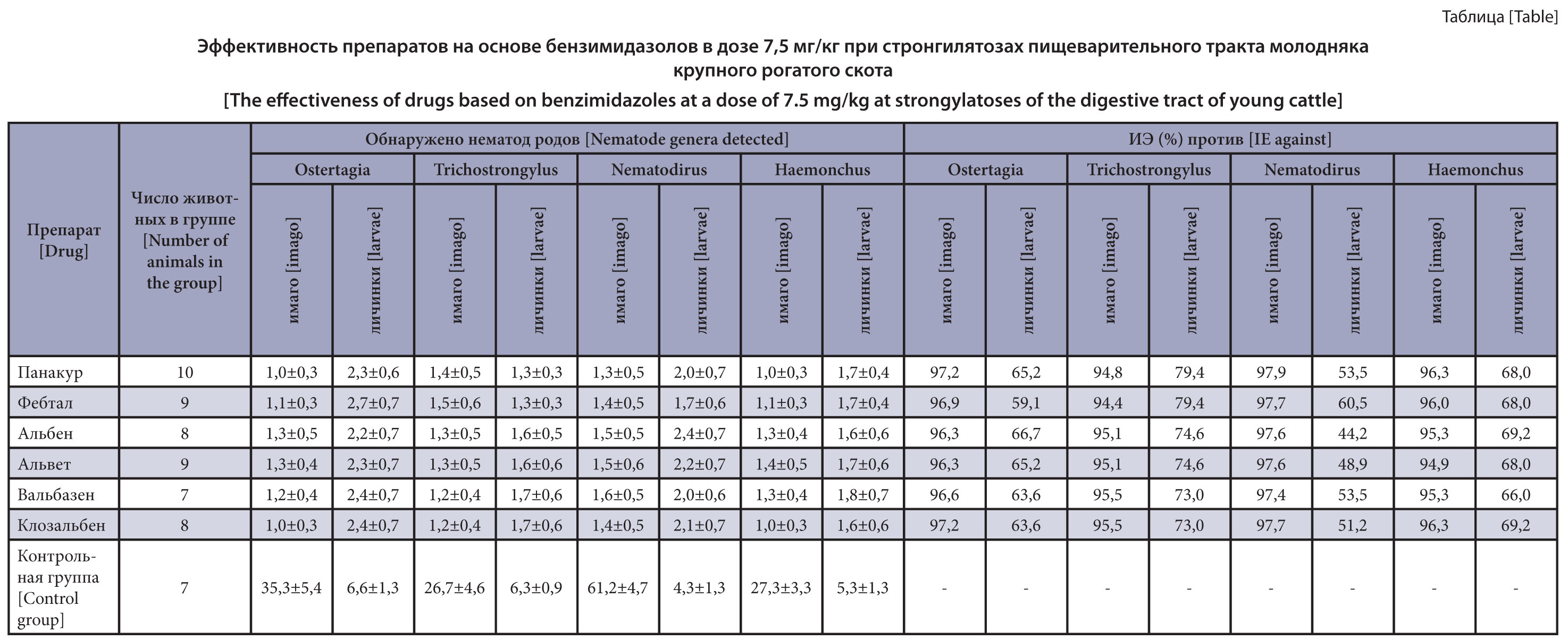
The purpose of the research is to study the efficacy of benzimidazole anthelmintics against different development stages of gastrointestinal nematodes of young cattle.
Materials and methods. The efficacy of benzimidazole drugs against early development stages of gastrointestinal nematodes was evaluated on 58 male calves aged 12–18 months spontaneously infected with gastrointestinal strongylates on the Moscow Region farms contaminated by nematode parasites. The animals were weighed, numbered and divided into experimental and control groups of 7–10 animals each. The male calves from different experimental groups were orally administered Panacur, Febtal, Fenbendazole (substance), Alben, Alvet, Valbazen, Closalben and Albendazole 10% powder once at a dose of 7.5 mg/kg for the active substance. The control animals did not receive the drug. The drug efficacy was recorded in the experiments of the "control test" type based on the coproovoscopic examination results by the flotation method using a VIGIS counting chamber, and on the results of helminthological dissections of the digestive tract of 3–5 animals from each group. The drug efficacy was recorded as per the Guidelines Approved by the World Association for the Advancement of Veterinary Parasitology (1995). The results were processed statistically using the Microsoft Excel computer tool.
Results and discussion. We established the 94.4–97.2% efficacy of the drugs based on benzimidazoles, namely, Panacur, Febtal, Alben, Alvet, Valbazen and Closalben in therapeutic doses against imaginal gastrointestinal strongylates and 44.2–69.2% activity against nematode larvae.
The purpose of the research is to develop and test new dosage forms against bothriocephalosis of cyprinids.
Materials and methods. The study was performed in the experimental therapy laboratory of the VNIIP – FSC VIEV, and on a cage fish farm of Cherepetsky Rybkhoz JSC (Suvorov, Tula Region). A formulation was developed for two micronized dosage forms of albendazole and praziquantel to prevent and treat cestodosis in fish, namely, micronized albendazole and micronized praziquantel. For experiments to test micronized dosage forms of the drugs against bothriocephalosis of cyprinids, medicated feeds were developed using a special technology with added hot water and beet molasses. Each medicated feed contained 2% of the praziquantel- and albendazole-based dosage form. To determine the infection of the fish with cestodes, carp yearlings were studied at Cherepetsky Rybkhoz JSC. Clinical examination and pathoanatomical dissection of the fish were conducted selectively using a common method, after which the prevalence and intensity of the Bothriocephalus sp. infection were determined in fish. The medicated feed was given at the rate of 5% of fish weight in the trays, and put into the feeders installed at the bottom of the trays. The first group of fish received the medicated food with micronized albendazole, and the second, with micronized praziquantel. The doses were 25 and 30 mg/kg for the active substance, respectively. The effectiveness of experimental batches of the medicated feeds with praziquantel and albendazole dosage forms was recorded by results of the helminthological dissection of all experimental and control fish on day 4 of the therapeutic feeding.
Results and discussion. Preliminary tests of the micronized albendazole and praziquantel dosage forms in the medicated feeds showed a 58.3% extense-effectiveness with a 61.9% intense-effectiveness, and a 75.0% extense-effectiveness with a 88.1% intense-effectiveness when applied once at a dose of 5% of fish weight (doses of 25 and 30 mg/kg for the AS, respectively).
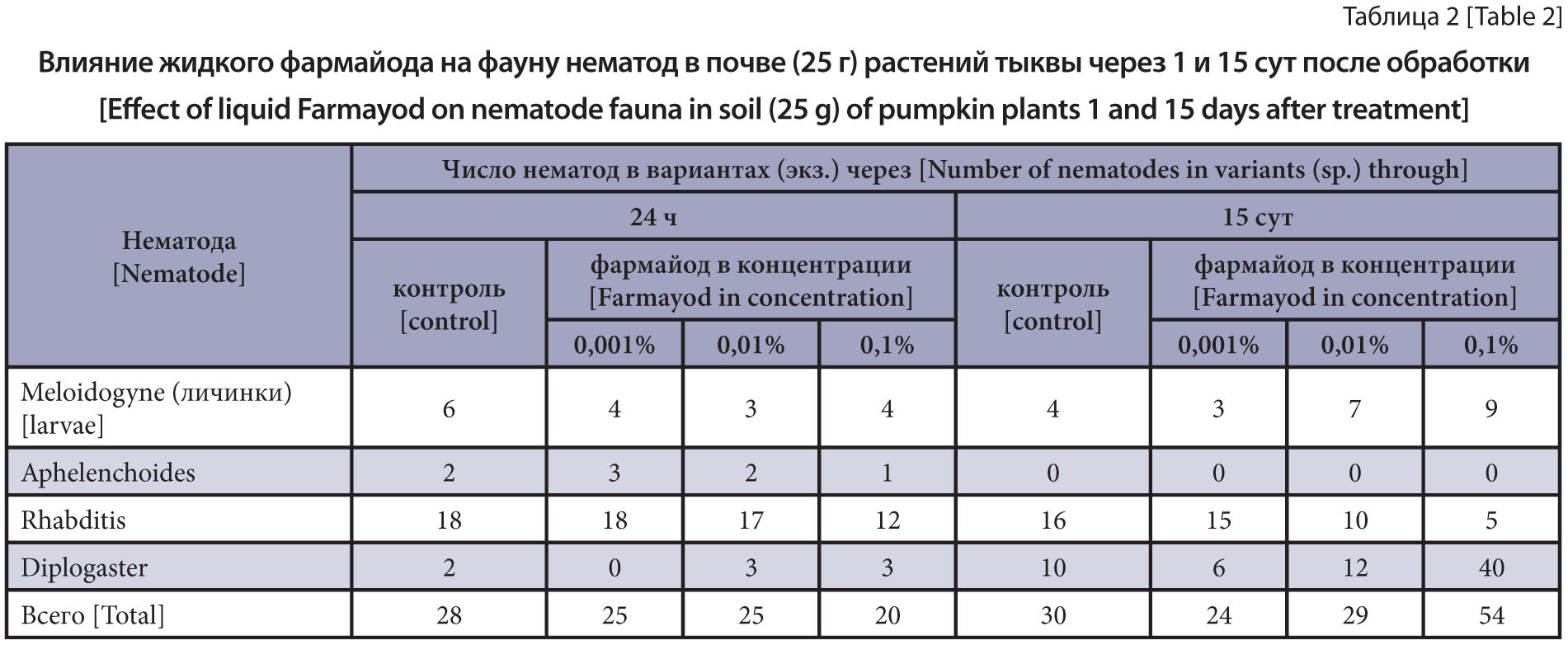
The purpose of the research is to study the effect of Farmayod on nematodes of different trophic groups, including rootknot nematode larvae, in vitro and in vivo.
Materials and methods. The object of the research were larvae of the root-knot nematode Meloidogyne incognita obtained from the roots of infected plants from the Vladimir Region. The study of the effect of Farmayod in three concentrations was carried out in laboratory, using the biotest method on pumpkin plants. The effect of liquid Farmayod on the viability of nematodes of different trophic groups was studied in vitro and in vivo.
Results and discussion. Liquid 0.1% Farmayod showed phytotoxicity, and not a single plant germinated. The drug in the form of a 0.01% solution showed phytotoxicity but to a lesser extent. The root system was less developed (60%) than in the control. The plant height was also 15% less. Farmayod at a concentration of 0.01% did not have phytotoxicity and reduced meloidoginosis versus the control. The biological efficacy of such dose was 56% higher, and the plant height was 30% more. The drug at a concentration of 0.01% had no effect on the plants damaged by meloidoginosis due to its phytotoxicity and poorly developed root system of the plants versus the control. Thus, at low concentrations, Farmayod acts on plants as a trace element necessary for plant vegetation, which affected the size of the plant. On the other hand, it can significantly reduce the infection of pumpkin roots with root-knot nematodes. Since this concentration is not toxic to nematodes, it can be assumed that the drug affects the nematode indirectly through the plant.
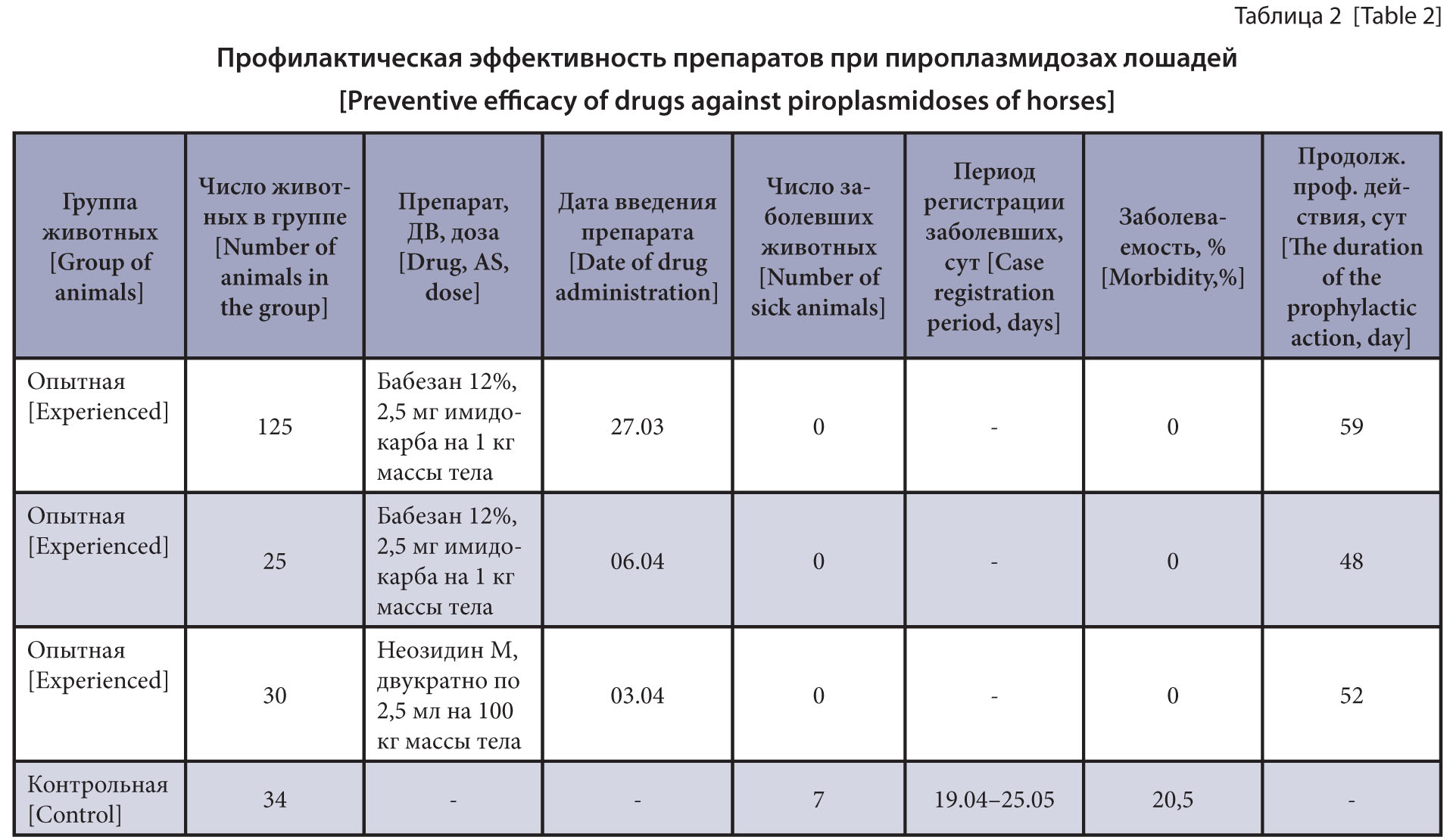
The purpose of the research is to study the prophylactic efficacy of piroplasmicides and to elucidate the effect of prophylactic doses of Imidocarb Dipropionate on the pathogen viability.
Materials and methods. Babezan 12% was intramuscularly injected to 150 horses based on the active substance of Imidocarb Dipropionate at 2.5 mg/kg of the body weight and Neosidin M was injected to 30 horses twice at a dose of 2.5 mL per 100 kg of the live weight with a 15-day interval. The control group of animals did not receive the drug. The clinical follow-up of the experimental and control animals’ condition was done for 72 days. Before the experiment and 14 days after the drugs, blood samples were examined by the nested PCR in the presence of genus-specific primers from the 18S rRNA gene sequence for the Babesia spp. / Theileria spp. DNA. Species identification and genotyping of the detected Piroplasma were performed by sequencing 18S rRNA gene fragments.
Results and discussion. Among 12 examined horses, 8 animals (66.7%) had the Piroplasmida DNA found in the blood samples, of which 50.0% were identified as Theileria equi and 16.7% as Babesia caballi. A high T. equi DNA prevalence (over 50.0%) indicates an endemic course of equine theileriosis. Babesan 12% in early chemotherapy of the horses helped to prevent the incidence of theileriosis within 48 and 59 days. Double chemotherapy of the horses with Neosidin M with a 15-day interval prevented the morbidity for 52 days. Babesan 12% at the preventive dose had no effect on the viability of persistent T. equi stages.
The purpose of the research is to determine safe parameters for the use of certain fatty acid amides against protozoal diseases of fish.
Materials and methods. The synthesis and biological activity determination of new compounds were studied in laboratory conditions of the chemical synthesis creative team of the North Caucasus Zonal Research Veterinary Institute. The protistocidal activity of the new substances was determined in a liquid nutritional medium by serial dilution according to our technique. The ciliate Colpoda steinii was used as a test culture. The solution toxicity was determined on aquarium Guppy (Poecilia reticulata). The efficacy was assessed according to an alternative form of the response: the minimum concentration that all protozoan species died in was considered as protistocidal; the maximum concentration that all fish remained alive in was recognized as safe for the fish, i.e., the first concentration after the absolute lethal concentrations. Compliance with these conditions required the prepared solutions having minimal concentration ranges from 1 to 0.1 µg/mL, as well as continuous monitoring with recording the results. Two compounds were tested, namely, myristic acid amide and oleic acid amide.
Results and discussion. The fish remained viable in the aqueous myristic acid amide solution concentration of 0.2 μg/mL for 3 days (monitoring period), and the fish remained alive for 3.5 hours or more (up to 20 hours) in the solution concentration of 0.5 μg/mL. At the same time, a concentration of 0.14 μg/mL had a protistocidal effect on the protozoan C. steinii if exposed for 40 minutes. The maximum tolerated oleic acid amide concentration for fish was 1 µg/mL if exposed for 2 or more days and 2 µg/mL if exposed for 4 h. The minimum protistocidal activity level of oleic acid amide was 0.58 µg/mL at an 18 h exposure.
OUR ANNIVERSARIES
ISSN 2541-7843 (Online)